Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Adsorption of Malachite Green Dye in Aqueous Solution Using Low-Cost Synthesis of Zinc Oxide Urea Formaldehyde Nanocomposite as Adsorbent
Authors: Prapti P. Warbhe, Rakesh M. Naktode, Mamta R. Lanjewar
DOI Link: https://doi.org/10.22214/ijraset.2024.63534
Certificate: View Certificate
Abstract
In recent decades, the world’s fast industrialization has made water contamination the most serious problem in the world. Organic dyes, especially malachite green, are synthetic dyes used in the textile industries to dye silk, cotton, leather, wool, paper, etc. and consequently discharged into the water resources that pollute aquatic environments. The purpose of the research work is to study the efficiency of zinc oxide urea formaldehyde nanocomposite used as an adsorbent in the removal of malachite green dye from polluted wastewater. The main stages of this work include the synthesis of zinc oxide by the simple sol-gel method at room temperature. The synthesised nanoparticle was coated with urea formaldehyde to increase its efficiency. The characterization techniques such as XRD, FTIR, SEM, and BET of the synthesised nanomaterials show that the zinc oxide urea formaldehyde nanocomposite was successfully synthesised. The batch adsorption study of the dye onto the ZnO-UF nanocomposite was investigated with a UV-visible spectrophotometer. The application of this synthesised nanocomposites as an adsorbent successfully adsorbed more than 97% malachite green dye at a reducing dye strength of 40 PPM, doses of 0.35 g, and pH 8 within a 90-min equilibration time.
Introduction
I. INTRODUCTION
Water is paramountly important on our planet. But in recent decades, water pollution has been common all over the world with the rapid increasing population and vast development in different industries such as textiles, leather dyeing, cosmetics, paints, plastics, paper manufacturing, etc., which used dyes for colouring their products and also used large amounts of water and chemicals and subsequently discharged large amounts of coloured waste water, which were not degraded and hence pernicious to environmental health[1], [2]. Different types of dyes are widely used in several textile industries because of their favourable characteristics, such as their intense colour, water-soluble nature, and handy application[3]. It is estimated that approximately 10–20% of the used dyes are released into the water resources, and these dyes and their breakdown products are very hazardous for all living beings, causing serious diseases and disorders[4]. Therefore, the removal of dyes from the industrial-coloured waste water is very important before it is discharged into the aquatic ecosystem[1], [4]. Among the many synthetic dyes, malachite green (MG) is a very toxic dye. Malachite green dye is widely used in the acrylic, food, paper, and paint industries. It is also used in aquaculture as a therapeutic agent[2], [5]. The high-water solubility of MG causes water pollution and can result in mutagenesis, carcinogenesis, teratogenesis, respiratory diseases, damage to the central nervous system, digestive system, liver, kidney, spleen, lung, heart, skin, bones, and brain[6]. It can also cause infertility issues. So, it is mandatory to remove MG from aquatic resources[2]. Many methods, such as biological treatment, ultrafiltration, ion exchange, coagulation, membrane separation, oxidation, adsorption, etc., are available for the removal of organic and synthetic dyes[1], [5], [7]-[10]. But the major drawbacks of many of these methods are that they require high sludge production, are expensive and potentially hazardous to the environment, require handling and lead to residual toxic species, etc. This necessitates low-cost, economically efficient, and low-energy-use techniques for the treatment of dyes present in industrial waste water[4], [11], [12] Metal oxide-based compounds such as zinc oxide have attracted great attention as adsorbent materials due to their excellent surface area and high adsorption capacity. It is non-toxic and chemically stable. Hence, it has been utilised for wide-ranging applications[9], [13]-[16]. In the present work, zinc oxide was synthesised by the sol-gel method and coated with urea formaldehyde polymer to increase its efficiency. The synthesised zinc oxide urea formaldehyde nanocomposites are characterised by various spectroscopic techniques such as SEM, XRD, FTIR, and BET. And applied as a low-cost adsorbent to the adsorption of malachite green dye from industrial waste water.
II. EXPERIMENTAL
A. Materials
Zinc Acetate Dihydrate (Zn (CH3COO)2.2H2O) ≥99% purity (Sigma Aldrich), Sodium Hydroxide (NaOH) ≥98% (Sigma Aldrich), Ethanol (CH2COOH) (analytical grade), and double distilled water. Zinc acetate dihydrate was used as a precursor, and ethanol was used as a reagent. Distilled water was used as a solvent medium. All the aqueous solutions were prepared using double-distilled water.
B. Synthesis of zinc oxide nanostructure
For the preparation of zinc oxide nanoparticles by using the sol-gel method, zinc acetate dehydrate was used as a precursor, sodium hydroxide was used as a precipitating agent, double-distilled water was used as the solvent medium, and ethanol was used as a reagent.
In order to prepare a sol, 20 g of zinc acetate dehydrate was dissolved in 150 ml of double-distilled water, and 80 g of NaOH was dissolved in 100 ml of double-distilled water. The solutions were stirred with constant stirring for about 10 minutes each. After being well mixed, sodium hydroxide solution was added dropwise. A large amount of white slurry was formed, and then a burette was filled with 500 ml of ethanol and titrate dropwise into the solution containing both sodium hydroxide solution and zinc acetate dihydrate. After a reaction, a white precipitate was formed.
C. Synthesis of Zinc oxide –Urea formaldehyde nanocomposite
20 ml of 40% formaldehyde and 10 g of urea were mixed in a molar ratio of 2:1 (w/w) in a 250 ml beaker and stirred magnetically at 60 °C for 20 min, so that urea was dissolved in formaldehyde. In this mixture, 100 mg of zinc oxide were added while stirring. After 5 minutes, 0.5 ml of concentrated H2SO4 was added with continuous stirring. A white precipitate was obtained, which was washed with distilled water, dried in an oven, and placed in desiccators.
D. Preparation of Dye Solution
The malachite green dye stock solution was made by weighing 1.00 g of powdered malachite green dye. The dye was transferred quantitatively into a 1 L measuring flask, which was then filled with distilled water to achieve a dye concentration of 1000 mgL-1 in the solution. The stock dye solutions (1000 mgL-1) were prepared separately and stored at 4 ? in distilled water. Dilution of the stock solutions with distilled water generated the working solutions.
E. Estimation of Dye concentration
The dye concentration and removal efficiency of ZnO-UF nanocomposite were estimated using a UV-Vis spectrophotometer (Schimadzu UV-Spectrophotometer, Model UV-1800). For the estimation of dye in synthetic effluent, absorbance was measured at 617 nm against blank, and malachite green was used as the standard effect of experimental parameters for malachite green dye removal using ZnO-UF nanocomposites.
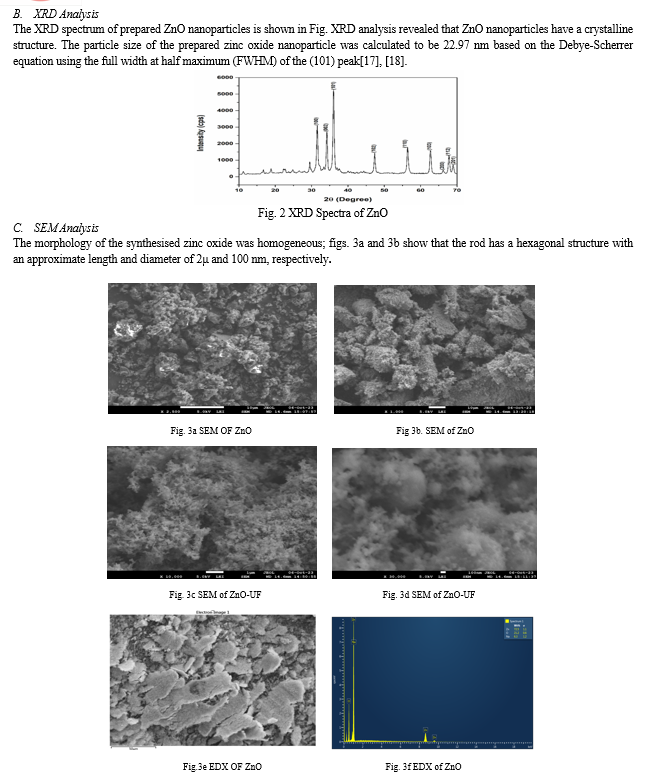
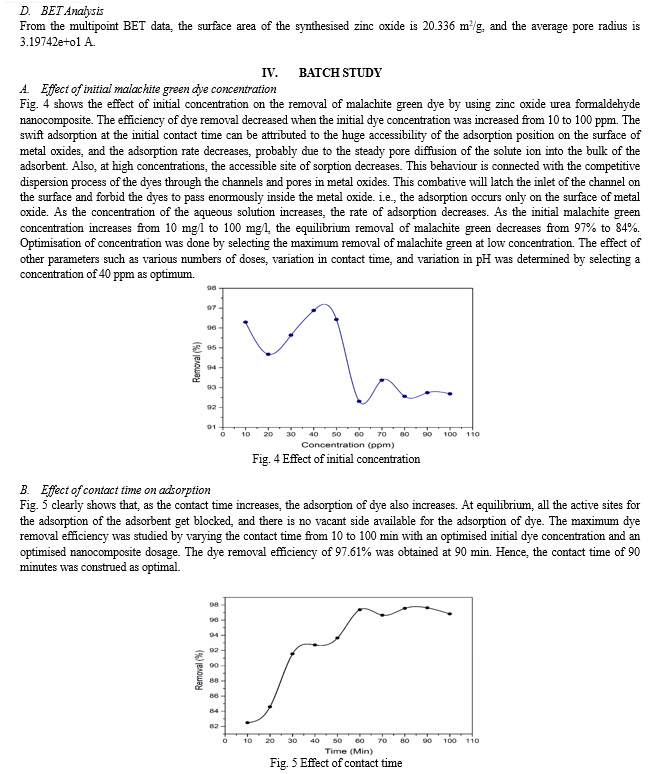
III. RESULT AND DISCUSSION
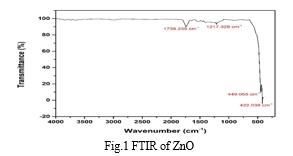
A. FT-IR Analysis
The characterization of nanostructured zinc oxide was carried out using a Bruker ALPHA-E FTIR spectrometer in the department of organic chemistry at RTM Nagpur University Nagpur. Bonding between ZnO is in the range of 400 to 700 cm-1. It means that the peak clearly represents the ZnO bonds.
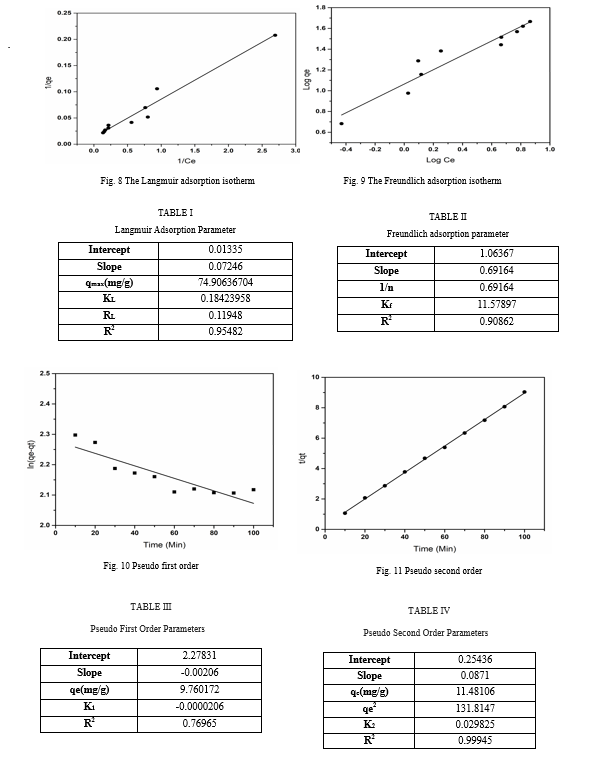
VI. ADSORPTION KINETIC
The kinetics of malachite green adsorption onto the zinc oxide urea formaldehyde surface were evaluated using different kinetic models, such as pseudo-first-order and pseudo-second-order. The varying parameters were calculated from the plots of the kinetic model equation (Tables 3 and 4). Among both of these models, the criteria for their applicability are the correlation coefficient (R2) and the concurrence between the calculated and experimental values of qe. The higher values of R2 ? 1 and near about the same experimental and calculated values of qe indicate that the system follows a pseudo-second-order kinetic model (tables 3 and 4).
Conclusion
In this work, the aim is to synthesise zinc oxide by the simple sol-gel method and characterise it. The synthesised zinc oxide is encapsulated with polymer urea formaldehyde in the ratio 1:2 to increase its efficiency. The nanocomposite zinc oxide urea formaldehyde provides an economically cheap, efficient, and eco-friendly approach for the removal of malachite green from wastewater. The studied initial malachite green concentration, adsorbent dose, contact time, and pH affected the adsorption yield notably. The Langmuir isotherm gave a better fit into the adsorption isotherm than the Freundlich adsorption isotherm. The kinetic study of malachite green on zinc oxide urea formaldehyde was performed based on pseudo-first order and pseudo-second order. The resultant data indicate the applicability of the pseudo-second-order kinetic model. The present study shows that due to the coating of urea formaldehyde on zinc oxide, the properties and applications of the present material changed drastically, and it was applicable to water remediation techniques.
References
[1] K. Y. Kumar, H. B. Muralidhara, Y. A. Nayaka, J. Balasubramanyam, and H. Hanumanthappa, “Low-cost synthesis of metal oxide nanoparticles and their application in adsorption of commercial dye and heavy metal ion in aqueous solution,” Powder Technol, vol. 246, pp. 125–136, Sep. 2013, doi: 10.1016/j.powtec.2013.05.017. [2] R. Sarathi et al., “5 Journal of Chemical Reviews Photocatalytic Degradation of Malachite Green Dye by Metal Oxide Nanoparticles-Mini Review Photocatalytic Degradation of Malachite Green Dye by Metal Oxide Nanoparticles-Mini Review,” J. Chem. Rev, vol. 5, no. 1, 2023, doi: 10.22034/JCR.2023. [3] A. A. Sabrin A, “Textile Dye Removal from Wastewater Effluents Using Chitosan-ZnO Nanocomposite,” J Text Sci Eng, vol. 05, no. 03, 2015, doi: 10.4172/2165-8064.1000200. [4] D. Nayeri and S. A. Mousavi, “Dye removal from water and wastewater by nanosized metal oxides - modified activated carbon: a review on recent researches,” Journal of Environmental Health Science and Engineering, vol. 18, no. 2. Springer Science and Business Media Deutschland GmbH, pp. 1671–1689, Dec. 01, 2020. doi: 10.1007/s40201-020-00566-w. [5] W. S. Al-Arjan, “Zinc Oxide Nanoparticles and Their Application in Adsorption of Toxic Dye from Aqueous Solution,” Polymers (Basel), vol. 14, no. 15, Aug. 2022, doi: 10.3390/polym14153086. [6] A. A. Alanazi, “Investigation of malachite green removal using graphene oxide-zinc oxide composite from aqueous solution: synthesis, characterization and application,” Desalination Water Treat, vol. 293, pp. 243–252, May 2023, doi: 10.5004/dwt.2023.29519. [7] S. Modi et al., “Recent and Emerging Trends in Remediation of Methylene Blue Dye from Wastewater by Using Zinc Oxide Nanoparticles,” Water (Switzerland), vol. 14, no. 11. MDPI, Jun. 01, 2022. doi: 10.3390/w14111749. [8] P. Saravanan, R. Gopalan, and V. Chandrasekaran, “Synthesis and Characterisation of Nanomaterials,” 2008. [9] V. Srivastava, D. Gusain, and Y. C. Sharma, “Synthesis, characterization and application of zinc oxide nanoparticles (n-ZnO),” Ceram Int, vol. 39, no. 8, pp. 9803–9808, Dec. 2013, doi: 10.1016/j.ceramint.2013.04.110. [10] V. M. Muinde, J. M. Onyari, B. Wamalwa, and J. N. Wabomba, “Adsorption of malachite green dye from aqueous solutions using mesoporous chitosan–zinc oxide composite material,” Environmental Chemistry and Ecotoxicology, vol. 2, pp. 115–125, Jan. 2020, doi: 10.1016/j.enceco.2020.07.005. [11] T. M. Tamer et al., “Formation of zinc oxide nanoparticles using alginate as a template for purification of wastewater,” Environ Nanotechnol Monit Manag, vol. 10, pp. 112–121, Dec. 2018, doi: 10.1016/j.enmm.2018.04.006. [12] Z. Monsef Khoshhesab and S. Souhani, “Adsorptive removal of reactive dyes from aqueous solutions using zinc oxide nanoparticles,” Journal of the Chinese Chemical Society, vol. 65, no. 12, pp. 1482–1490, Dec. 2018, doi: 10.1002/jccs.201700477. [13] R. Saravanan, V. K. Gupta, T. Prakash, V. Narayanan, and A. Stephen, “Synthesis, characterization and photocatalytic activity of novel Hg doped ZnO nanorods prepared by thermal decomposition method,” J Mol Liq, vol. 178, pp. 88–93, Feb. 2013, doi: 10.1016/j.molliq.2012.11.012. [14] R. Saravanan et al., “ZnO/Ag nanocomposite: An efficient catalyst for degradation studies of textile effluents under visible light,” Materials Science and Engineering C, vol. 33, no. 4, pp. 2235–2244, May 2013, doi: 10.1016/j.msec.2013.01.046. [15] R. Saravanan et al., “ZnO/Ag/CdO nanocomposite for visible light-induced photocatalytic degradation of industrial textile effluents,” J Colloid Interface Sci, vol. 452, pp. 126–133, Aug. 2015, doi: 10.1016/j.jcis.2015.04.035. [16] R. Saravanan, V. K. Gupta, V. Narayanan, and A. Stephen, “Comparative study on photocatalytic activity of ZnO prepared by different methods,” J Mol Liq, vol. 181, pp. 133–141, May 2013, doi: 10.1016/j.molliq.2013.02.023 [17] M. Ghaedi, F. N. Azad, K. Dashtian, S. Hajati, A. Goudarzi, and M. Soylak, “Central composite design and genetic algorithm applied for the optimization of ultrasonic-assisted removal of malachite green by ZnO Nanorod-loaded activated carbon,” Spectrochim Acta A Mol Biomol Spectrosc, vol. 167, pp. 157–164, Oct. 2016, doi: 10.1016/j.saa.2016.05.025. [18] J. N. Hasnidawani, H. N. Azlina, H. Norita, N. N. Bonnia, S. Ratim, and E. S. Ali, “Synthesis of ZnO Nanostructures Using Sol-Gel Method,” Procedia Chem, vol. 19, pp. 211–216, 2016, doi: 10.1016/j.proche.2016.03.095.
Copyright
Copyright © 2024 Prapti P. Warbhe, Rakesh M. Naktode, Mamta R. Lanjewar. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET63534
Publish Date : 2024-07-02
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

